The EpiPen Controversy Signals Intensifying Scrutiny of Drug Classification Under Medicaid Rebate Program
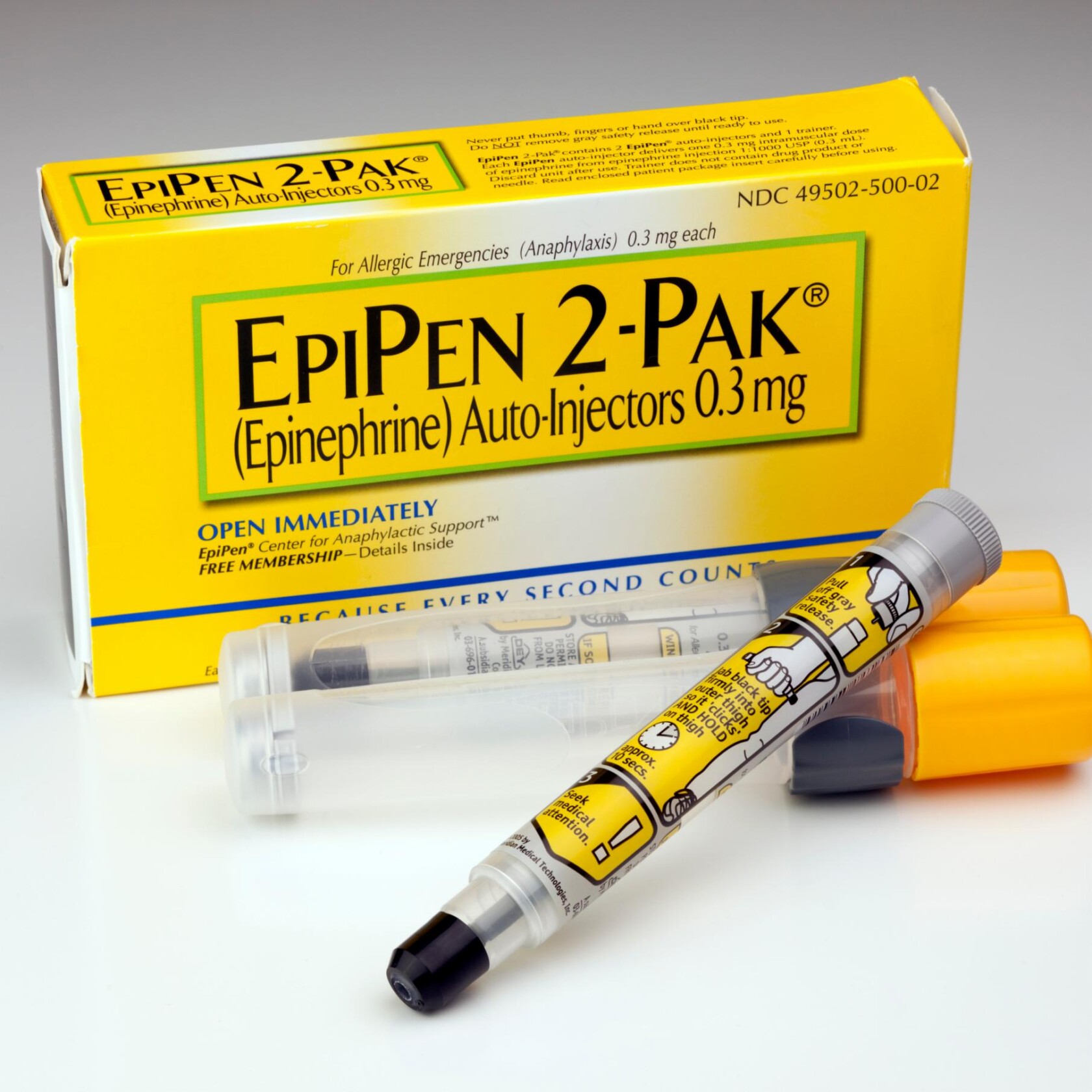
Price increases threatening the availability of EpiPen® and EpiPen Jr® Auto-Injectors (“EpiPen”) have touched off the latest firestorm over drug pricing. Lost amid the public outcry, however, is a thorny regulatory issue: EpiPen’s classification as a generic drug for purposes of the Medicaid Drug Rebate Program (“MDRP”). Resolution of the classification issue carries significant risk for segments of the drug industry.
EpiPen’s Backstory and the MDRP
EpiPen is an epinephrine auto-injector for emergency treatment of anaphylaxis. The U.S. Food and Drug Administration (“FDA”) approved EpiPen in December 1987 and currently lists it in the FDA National Drug Code Directory under a New Drug Application by its manufacturer, Mylan Specialty L.P. (“Mylan”). Following year-over-year price increases since Mylan acquired EpiPen in 2007, members of Congress now claim EpiPen originally was misclassified as a generic drug under the MDRP. Drug classification is significant, because generic drugs are subject to a substantially lower rebate than their brand counterparts, as explained in more detail below.
By way of background, Congress established the MDRP in 1990 as part of the Omnibus Budget Reconciliation Act, legislation enacted to help control the cost of Medicaid prescription drug coverage. For a drug to be eligible for Medicaid reimbursement, its manufacturer must enter an agreement with the U.S. Department of Health and Human Services (“HHS”) to pay rebates to states based on the utilization of the drug. The agreement requires manufacturers to report, on a quarterly basis, statutorily defined pricing information (i.e., Average Manufacturer Price (“AMP”) and best price) that is used to calculate the rebate the manufacturer must pay. Currently, the rebate for single source and innovator multiple source drugs is the greater of 23.1% of the AMP per unit or the difference between the AMP and the best price per unit. For non-innovator, multiple source drugs, the rebate is 13% of the AMP per unit. Further, since the passage of the Affordable Care Act in 2010, generic drugs (i.e., non-innovator multiple-source drugs) been exempt from the additional inflationary rebate applied to brand drugs for which AMP increases faster than the rate of inflation.
In October 1997, the Health Care Financing Administration (“HCFA”), now known as the Centers for Medicare & Medicaid Services (“CMS”), conferred with FDA “in order to determine the Drug Category . . . to use when reporting [EpiPen]” to the MDRP. “Because these products are included in a package with a new delivery system, they are listed by the FDA under an NDA (New Drug Application),” HCFA stated; however, “[t]he products themselves . . . are listed under an ANDA (Abbreviated New Drug Application) because they are very old products and made by many generic drug companies.” HCFA concluded, “even though the current NDCs of these products . . . are listed under an NDA, it is entirely fitting and proper . . . to report them to the Drug Rebate Program with a Drug Category of ‘N’ (Non-innovator, Multiple Source) and be subject to the lowest rebate amount of 11% of quarterly AMP.” See id. (emphasis added). EpiPen has since been designated as a non-innovator drug in calculating federal rebates.
Legislators have seized on the 1997 letter, voicing frustration directly to HHS and CMS. On August 30, 2016, a bipartisan group of U.S. Senators called on CMS “to explain why EpiPens are considered a non-innovator multiple source drug rather than an innovator multiple source drug.” On September 2, Ranking Members of the Senate Finance Committee and House Energy & Commerce Committee wrote to HHS complaining EpiPen’s generic classification “is inconsistent with how the FDA lists the EpiPen®, as well as how Medicare treats these products for purposes of the Medicare Part D prescription drug program. As a result, it appears that Medicaid may have been grossly overpaying for EpiPen® and its related products due to Mylan’s misclassification,” because of lower Medicaid rebate payments on EpiPen.
EpiPen’s Regulatory Conundrum
Whether and how EpiPen actually was misclassified as a generic drug presents a novel and important legal question. On the one hand, EpiPen’s active pharmaceutical ingredient (“API”), epinephrine (or adrenaline), is a non-innovator medication and hormone approved for sale under an abbreviated NDA in May 1984. On the other hand, Mylan’s EpiPen is marketed under an NDA because it uses a patented auto-injector to intravenously deliver a measured dose of epinephrine.
And EpiPen hardly is alone. As The New York Times reported before the Labor Day holiday, “[m]any other old medications have been delivered in new packages in recent years.” These include asthma inhalers and insulin injectors, plus emergency rescue drugs similar to EpiPen like GlucaGen® (indicated for treatment of severe hypoglycemia) and Narcan® (used for emergency treatment of opioid overdose), both of which utilize generic APIs in dosage forms administered by intramuscular injection using new types of auto-injectors. How should these products and others like them be classified for purposes of the MDRP?
According to CMS’s new Medicaid-covered outpatient drugs final rule, “[a]ll drugs marketed under an NDA, other than an ANDA, regardless of when they were approved, should be categorized as single source or innovator multiple source drugs, unless CMS determines that a narrow exception applies as discussed [] pursuant to this final rule.” 81 Fed. Reg. 5170, 5193 (Feb. 1, 2016) (emphasis added). Specifically, CMS acknowledged “[t]here may be very limited circumstances where, for the purposes of the Medicaid Drug Rebate (MDR) program, certain drugs might be more appropriately treated as if they were approved under an ANDA and classified as a non-innovator multiple source drug.” Id. at 5191. For example,
certain parenteral drugs in plastic immediate containers, for which FDA required that an NDA be filed, might be more appropriately treated, for purposes of the MDR program, as if they are marketed under an ANDA and classified as a non-innovator multiple source drug. Likewise, certain drugs approved under a paper NDA prior to the enactment of the Hatch-Waxman Amendments of 1984 or under certain types of literature-based 505(b)(2) NDA approvals after the Hatch-Waxman Amendments of 1984 might be more appropriately treated as if they were approved under an ANDA and classified as a non-innovator multiple source drug, depending on the unique facts and circumstances of the particular situation.
Id. at 5191. Under the rule, “[t]o the extent a manufacturer has previously reported a drug [like EpiPen] marketed under an NDA, other than an ANDA, as a non-innovator multiple source drug, or believes it has approval from CMS to do so, that manufacturer must submit materials and receive a written determination from CMS as described above pursuant to this final rule.” Id. at 5192.
More recent agency guidance muddies the analysis, however. According to a May 2 CMS manufacturer release relating to the MDRP, “manufacturers may not rely upon previous communications from CMS regarding Drug Category reporting that were provided prior to the publication of the Final Rule to justify their classification of a drug marketed under an NDA as a non-innovator multiple source drug.” (emphasis added). Notably, too, “the determination as to whether the narrow exception applies for the classification of the drug will depend on the drug itself and NDA at issue, not the active ingredient in the drug.” Id.
We understand that Mylan is standing by the decision to classify EpiPen as a non-innovator drug and plans to apply for continued non-innovator status by the final rule’s April 1, 2017 deadline. While the fate of Mylan’s bid to avoid reclassifying EpiPen is uncertain, the controversy surrounding EpiPen likely will spur government investigations and enforcement actions over whether drug products reported as non-innovator multiple source drugs were fraudulently or improperly misclassified to lower rebates to Medicaid. We will report on developments in this area.


