
On December 12, 2019, an outbreak of a severe pneumonia broke out of Wuhan, China. The causative agent of the outbreak was determined to be a coronavirus, an enveloped positive- and single-stranded RNA virus that infects vertebrates. The newly discovered coronavirus belongs to the severe acute respiratory syndrome related coronavirus (SARS-CoV)1 species. The new infectious respiratory syndrome is now known as the Coronavirus Disease 2019 (COVID-19).2
Since the declaration of a public health emergency on February 4, 2020, the FDA has authorized the emergency use of about one hundred (100) SARS-CoV-2 molecular diagnostic tests for use under certain conditions; thirty seven (37) molecular laboratory developed tests (LDTs) for detection of SARS-CoV-2 that meet specified criteria for eligibility under the umbrella emergency use authorization; two (2) antigen diagnostic tests; twenty-five (25) serology/antibody tests; one in vitro diagnostic test for the management of COVID patients, and three (3) drug products for the treatment of COVID-19 or the management of COVID patients.3
While currently the deadliest coronavirus infectious disease to date4 with 13,132,491 people infected and 573,834 dead worldwide, COVID-19 is not the first global SARS-CoV-mediated infection. In November 16, 2002, a severe acute respiratory syndrome (SARS), caused by the SARS coronavirus (SARS-CoV), emerged in Guangdong, China, and infected 8,000 people in 37 countries and killed 774. Then in June 2012, a Middle East Respiratory Syndrome (MERS) caused by the MERS coronavirus (MERS-CoV) emerged in Saudi Arabia and infected 2,494 people and killed 858. The causative agent of COVID-19, SARS-CoV-2 is only moderately similar to SARS-CoV1 (~79.6%) and MERS-CoV (~50%).5
Since, COVID-19 is not the first exposure to the deadliness of the SARS-CoV infection and in light of the rapidity with which diagnostic tests and drug products were approved for emergency use, this article explored prior research and innovation in SARS-CoV-mediated coronavirus diagnostic and therapies through patenting trends prior to the current COVID-19 pandemic. The number of patents and published patent applications relating to the prevention, treatment, diagnosis, protection from or alleviation of symptoms of SARS-CoV was used as a useful barometer to assess innovation to fight SARS-CoV. These patents and patent publications suggest that previous SARS-CoV outbreaks spurred innovation related to the diagnosis, and treatment of COVID-19.
Patent Search
| Countries | Number of Applications |
| WIPO | 240 |
| China | 616 |
| Europe | 189 |
| Japan | 179 |
| South Korea | 169 |
| United States | 599 |
A systemic worldwide search of patent databases for coronavirus or severe acute respiratory syndrome coronavirus or “SARS-CoV-2” or “sars cov” or “COVID-19” identified more than 10,326 patent publications, which were collapsed into 2,652 simple patent application families.6 Among these 2,652 publications, 599 applications were published in the United States. The breakdown of the patent applications among the largest Intellectual Property Offices is shown in the Table.
SARS-Cov Global Epidemic of 2002 Spurred Innovation
Among the 599 U.S. patent publications, 287 were issued patents (including 3 reissue), and 312 published applications. Figure 1 illustrates the annual distribution of these U.S. patent publications. The data show that patents related to coronaviruses have been published since at least 1975.  However, this number only started to increase after the first SARS-CoV outbreak and this rise has continued to this day7 (Figure 1). Among the 287 issued patents, only 55 patents were specifically drawn to SARS-CoV, MERS-CoV, human coronavirus NL63 (HCoV-NL63)8, or CoV-HKU1.9 These data suggest that previous SARS-CoV outbreaks spurred innovation related to the prevention, treatment, diagnosis, protection from or alleviation of symptoms of COVID-19.
However, this number only started to increase after the first SARS-CoV outbreak and this rise has continued to this day7 (Figure 1). Among the 287 issued patents, only 55 patents were specifically drawn to SARS-CoV, MERS-CoV, human coronavirus NL63 (HCoV-NL63)8, or CoV-HKU1.9 These data suggest that previous SARS-CoV outbreaks spurred innovation related to the prevention, treatment, diagnosis, protection from or alleviation of symptoms of COVID-19.
Early Sars-CoV Patents
(a) Diagnostic Patents
U.S. Patent No. 7,129,042 (“The ‘042 Patent”) issued in 2006 and was filed on November 3, 2003. The ‘042 Patent is directed to compositions and methods for detecting the presence of SARS-coronavirus proteins or genomic RNA in a sample and for screening anti-SARS coronavirus agents and vaccines.10 The claims were broadly written and an illustrative claim recites:
1. A method for detecting the presence of severe acute respiratory syndrome coronavirus (SARS-coronavirus) in a sample, comprising:
a) providing:
(i) a sample; and
(ii) cells chosen from human embryonic kidney (HEK)-293, HEK-293T, Huh-7, Mv1Lu, Mv1Lu-hf and primary rhesus monkey kidney (pRHMK) cells;
b) inoculating said cells with said sample to produce inoculated cells; and
c) detecting the presence of said SARS-coronavirus in said inoculated cells, wherein said detecting comprises detecting the presence of SARS-coronavirus subgenomic RNA.
The specification of the ‘042 Patent discloses the genomic RNA sequences of SARS-CoV TOR-2, Urbani, and CUHK-W1 strains. The specification also discloses nucleotide and amino acid sequences of specific genes such as orf1a and orf1ab polyprotein, Spike glycoprotein, Orf3a, Orf4b, Orf6, Orf7a, Orf7b, Orf8A, Orf8b, Nucleocapsid protein, envelope protein E, and membrane glycoprotein M. The ‘042 Patent, which has an anticipation expiration date of November 3, 2011, expired December 3, 2018 for failure to pay the maintenance fee.
U.S. Patent No. 7,129,223 (“the ‘223 Patent”) was filed on May 19, 2004 and issued on October 31, 2006. The ‘223 Patent claims priority to a provisional application filed on May 19, 2003, during the global epidemic. The patent is directed to the use of RNA interference (RNAi) molecules against the replicase region of the SARS-CoV virus for the treatment of SARS-CoV-mediated infection and vaccines and kits comprising these RNAi molecules. The two independent claims recite:
1. An isolated nucleic acid molecule consisting of the nucleotide sequence of SEQ ID NO:4 and/or the complement of SEQ ID NO:4.
8. A method for inhibiting SARS viral infection or replication in a cultured cell comprising administering to the cell an effective amount of the nucleic acid molecule consisting essentially of the nucleotide sequence of SEQ ID NO:4 and/or the complement of SEQ ID NO:4, or at least 10 or more contiguous nucleotides of the nucleotide sequence of SEQ ID NO:4 and/or the complement of said contiguous nucleotides.
The specification of the ‘223 Patent discloses the nucleic acid sequences of siRNAs targeting six different sites of the replicase 1A region (SEQ ID NO: 1-6), and siRNAs targeting the spike glycoprotein (S) region, the envelop protein (E) region, the nucleoscapsid protein (N) region, and the membrane protein (M) region. The ‘223 Patent has an anticipated expiration date of May 19, 2024. Other relevant patents drawn to RNAi or antisense oligonucleotides targeted to SARS-CoV genes for use as antisense therapy include U.S. Patent Nos. 7,339,051; US 7,429,656; and 7,553,944.
U.S. Patent No. 7,374,883 (“The ‘883 Patent”) was filed on April 30, 2004, issued on May 20, 2008, and claims priority to U.S. provisional application No. 60/467,117 filed on April 30, 2003. The patent is directed to oligonucleotides and kits for the sensitive and reliable quantitative real time PCR method of detecting SARS-CoV nucleic acid in biological samples. The ‘883 Patent has an anticipated expiration date of October 17, 2024.
Other patents that disclose and/or claim various SARS-CoV proteins that can be used for diagnosing SARS-CoV infection or for inducing an antigenic and/or immunological response include U.S. Patent Nos. 7,279,327; 7,361,747; 7,371,837; 7,491,397; 7,504,205; 7,740,858; 7,393,638; and 7,582,740.
(b) Inhibitory Compounds
The first SARS-CoV-directed patent uncovered in the search is U.S. Patent No. 7,048,953 (“The ‘953 patent”), which issued on May 23, 2006. The ‘953 patent claims concentrated vapors from botanical essential oils for the treatment or prevention of SARS-CoV-related infection, an inhaler for administering the botanical essential oil, and a method of reducing SARS-CoV infection using inhaled concentrate vapors from botanical essential oils. This patent is notable because it shows how innovators extended existing data to a new technological field.
The ‘953 patent was filed on May 2, 2003, six months after the 2002 SARS-CoV outbreak started in China, and three months after the World Health Organization issued a global health alert concerning the outbreak.11 The ‘953 patent is a continuation-in-part of a U.S. patent application No. 10/269,891 (filed on October 12, 2002); and claims priority to two provisional applications that were filed before the first SARS outbreak, and four provisional applications that were filed within weeks of the WHO press release.
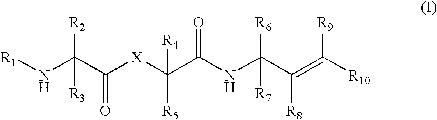 U.S. Patent No. 7,462,594 was filed on February 25, 2005, issued on December 09, 2008, and claims priority to a provisional application filed on December 31, 2003. The claims of the patent are directed to peptide-like compounds of formula I (shown to the right) that inhibit coronaviral 3CL proteases or flaviviridae viral proteases for the treatment of coronavirus or a flaviviridae virus infection.
U.S. Patent No. 7,462,594 was filed on February 25, 2005, issued on December 09, 2008, and claims priority to a provisional application filed on December 31, 2003. The claims of the patent are directed to peptide-like compounds of formula I (shown to the right) that inhibit coronaviral 3CL proteases or flaviviridae viral proteases for the treatment of coronavirus or a flaviviridae virus infection.
The application that issued as U.S. Patent No. 10,695,361 was filed about four months before COVID-19 broke out of Wuhan, China. The patent claims priority to two provisional applications filed on September 16, 2015, and October 9, 2015. This specification discloses methods and compounds for treating Arenaviridae virus and Coronaviridae virus infections; in particular, methods and nucleosides and prodrugs for treating Lassa virus, Junin virus, SARS virus and MERS virus. Illustrative claims of the patent include:
1. A. Method for treating a Coronaviridae infection in a human in need thereof, comprising administering a therapeutically effective amount of a compound of Formula IV
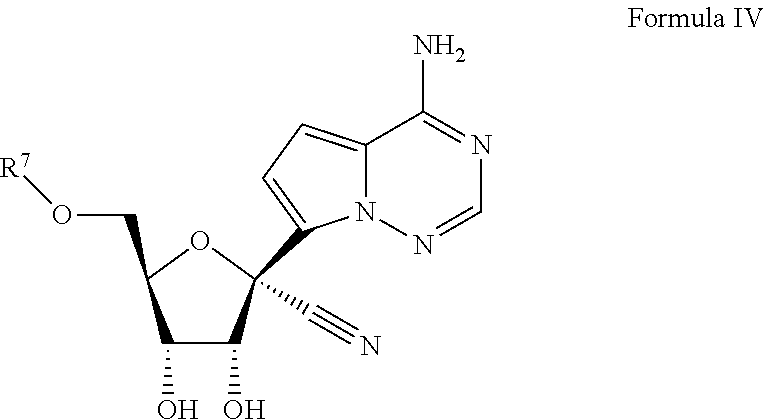
or a pharmaceutically acceptable salt thereof . . . .
19. The method of claim 1, wherein the Coronaviridae infection is caused by a Coronaviridae virus selected from SARS, MERS, 229E, NL63, OC43, and HKU1.
20. The method of claim 1, wherein the compound of Formula IV is
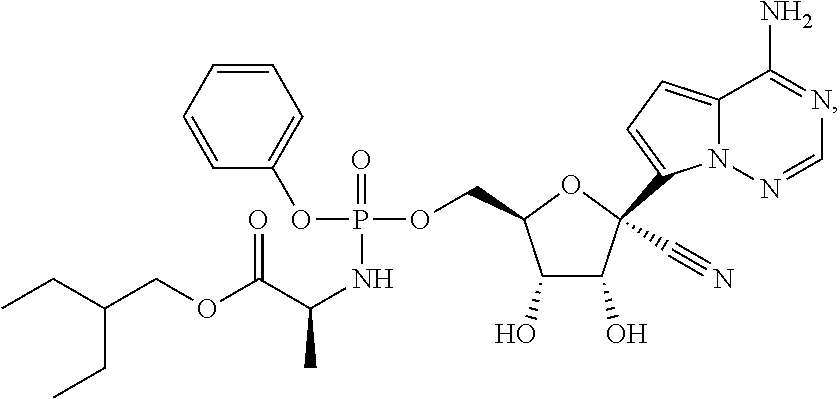
or a pharmaceutically acceptable salt thereof.
Another patent of note is U.S. Patent No. 7,504,382 that was filed on May 6, 2004 and claims priority to a provisional application filed on May 6, 2003. The patent claims are directed to inhibitors of SARS-CoV and coronaviruses, or picornaviruses proteases, and their use for the treatment or amelioration of diseases caused by coronaviruses, SARS-CoV, or picornaviruses.
The search of the patent literature also identified patents directed to compounds for the treatment of SARS-CoV infection and include: U.S. Patent Nos. 7,504,382; 10,246,486; 7,605,135; 8,481,571; 10,143,709; 9,975,885; and 10,307,439, and U.S. Patent Publication Nos: 2019/0285632A1; 2019/0314497A; 2019/0336456A1; 2019/0337981A1; and 2019/0389816.
High Degree of Abandonment
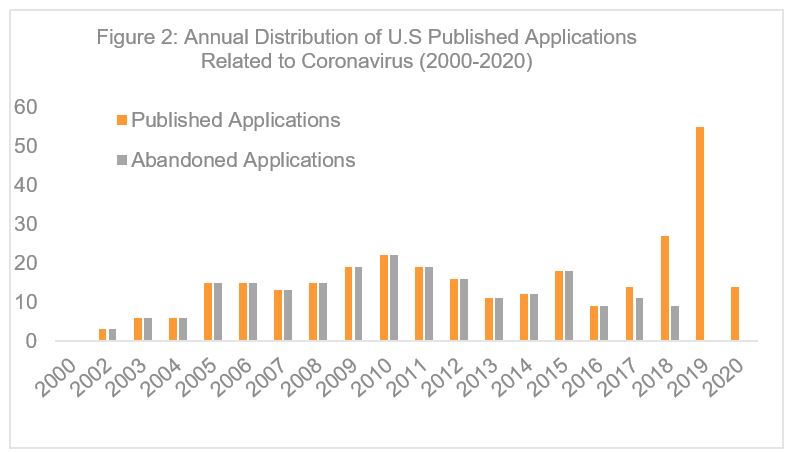 The data also show that most early patent applications related to SARS-CoV did not ultimately issue. In particular, 219 of the 312 identified published applications were abandoned (Figure 2). An evaluation of 25 randomly selected abandoned applications shows that 12 applications were abandoned before any substantive examination. The remaining applications were abandoned for failure to respond to an office action, in which the claims were alleged to lack clarity (6), written description (6), enablement (3), novelty (8) and for allegedly being obvious over a prior art (8). Four of these applications contains obviousness-type double patenting rejections.
The data also show that most early patent applications related to SARS-CoV did not ultimately issue. In particular, 219 of the 312 identified published applications were abandoned (Figure 2). An evaluation of 25 randomly selected abandoned applications shows that 12 applications were abandoned before any substantive examination. The remaining applications were abandoned for failure to respond to an office action, in which the claims were alleged to lack clarity (6), written description (6), enablement (3), novelty (8) and for allegedly being obvious over a prior art (8). Four of these applications contains obviousness-type double patenting rejections.
This high rate of abandonment does not diminish the impact that those disclosures may have had on spurring current innovations. Many of these publications disclose specific biomarkers that could be used to diagnose SARS-CoV infection, and/or SARA-CoV antigen for a vaccine. These abandoned applications contributed directly or indirectly to the current knowledge base for the current COVID-19 pandemic, and may have provided or could still provide technology that could be explored for use in diagnosing and treating COVID-19 without the need for licensing or fears of infringement liability.
Innovation Continues With USPTO Support
COVID-19 creates a spectrum of symptoms that range from none, to mild, medium, and severe symptoms. This range of symptoms appears to be dictated by a person’s owned immune response. COVID-19 appears to have two phases. A first phase that is or may be responsive to antiviral drugs, and a second phase that requires modulating and moderating the immune response to either prevent an hyper-immune response (cytokine storm) or to temper it once it is underway.12 Therefore fighting the illness requires not just coming up with the best and most effective anti-viral or anti-SARS-CoV-2 drugs, but also determining how to manage a person’s immune response to prevent the so called cytokine storm. Therefore, innovation for fighting transient infectious diseases requires casting a wide net.
When COVID-19 was declared a global pandemic in March 2020, the World Health Organization (WHO) looked to research and innovation to temper and overcome the virus. The U.S. government also took swift action. The United States Patent and Trademark Office (USPTO) implemented new measures to accelerate innovation related to COVID-19. In particular, the USPTO implemented prioritized examination for COVID19 related inventions13, and launched the Patents 4 Partnerships IP marketplace platform to facilitate the voluntary licensing and commercialization of innovations” related to “the prevention, treatment, and diagnosis of COVID-19.”14
With the public health need and support from the government and USPTO, we expect the number of published application related to the treatment, prevention or diagnosis of COVID-19 to spike in about 16 months, or 18 months from the initial outbreak. To the benefit of the public, we hope that each, and every filing provides a benefit to the eradication or cure of this disease.
——————————————————
1 Lu et al., Lancet 395: 565–574 (2020).
2 WHO Director-General, Remarks at the media briefing on 2019-nCoV (WHO, 11 February 2020), https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020</a> ; Gorbalenya, A. E. et al. (bioRxiv (Feb. 7, 2020) https://www.biorxiv.org/content/10.1101/2020.02.07.937862v1 .
3 Coronavirus Disease 2019 (COVID-19) EUA Information, https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#covid19euas.
4 COVID-19 Case Tracker (July 14, 2020,9:06 AM), https://coronavirus.jhu.edu/.
5 Zhou et al., Nature 579, 270–273 (2020).
6 Patent Search was conducted by Robin S. Evans, Foley & Lardner, LLP.
7 Search was conducted on April 4, 2020.
8 Van der Hoek et al., Nat Med. 10(4):368-73 (2004); Fouchier et al. Proc Natl Acad Sci 101(16):6212-6 (2004).
9 Woo et al., J. Virol. 79(2):884-95 (2005).
10 Gillim-Ross et al., U.S. Patent No. 7,129,042, Compositions and Methods for Detecting Severe Acute Respiratory Syndrome Coronavirus, filed November 3, 2003. The patent is nominally assigned to Diagnostic Hybrids, Inc. (Athens, OH, US) and Health Research Incorporated (Rensselaer, NY, US).
11 Press Release, WHO, Acute respiratory syndrome in Hong Kong Special Administrative Region of China/ Viet Nam(March 12, 2003), https://www.who.int/csr/don/2003_03_12/en/; https://www.who.int/csr/don/2003_03_16/en/.
12 Pawlitzki et al., EBioMedicine 56:102822 (2020).
13 USPTO, PTO-P-202-0026, COVID-19 Prioritized Examination Pilot Program (2020), https://www.uspto.gov/sites/default/files/documents/COVID-19%20Prioritized%20Examination%20Pilot%20Program%2007May2020.pdf</a>;
14 Brinckerhoff C, New Platform to Facilitate Development of COVID-19 Technologies (May 6, 2020), https://www.foley.com/en/insights/publications/2020/05/platform-facilitate-development-covid-19-tech.
