
Advancements in the medical device space are increasingly driven by integrations of software, oftentimes as the fruit of the symbiosis between engineers at software development firms working with clinicians at research institutions. These technologies have the potential to improve the quality of care provided by doctors and the treatment outcomes and lives of patients. The implementation of artificial intelligence (AI) models for various health care and life sciences solutions is of particular interest for many players in this field. The market for AI in health care alone is projected to grow ten-fold to $100 billion in the next five years.
Growth through software necessitates that stakeholders recognize the value of their intellectual property (IP), which includes patents, copyright, trade secrets, and data rights in protocols, data sets, study results, etc. IP owners must readily leverage their assets in the context of co-development to ensure that value is maximized but also protected. SaMD frequently involve multiple stakeholders, each with varying interests and concerns regarding the current and future deployment of the technology.
Competing Stakeholder Interests in the Use and Deployment of AI
Consider a hypothetical (albeit common) scenario in which the interests and concerns of these stakeholders collide: the use and deployment of an AI model. AI may involve computer-implemented technology that attempts to mimic some aspect of human intelligence. Training data is used to teach an underlying model so that the resulting software can make reliable and accurate inferences with respect to newly-acquired data.
In the example shown above, there are three stakeholders that collaborate to deploy and use an AI model in a piece of SaMD:
- Company A, a software development firm that created the AI model using certain data;
- Company B, a research institute or hospital providing additional data that will be used for training the AI model; and
- Company C, a customer (e.g., a commercialization entity, a pharmaceutical manufacturer, insurance company, end users, etc.) making use of the AI model, either as part of a software as a service architecture or for use in connection with a medical device.
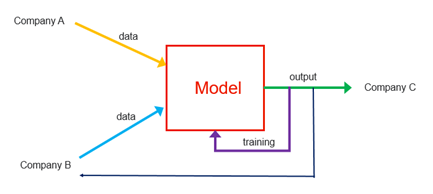
While one company may have in fact written the code to initially build the AI model, it is the collaboration among these companies that helped drive the AI model to fruition, ultimately generating value and improving the quality of care. The research institute, with the provision of data to train and refine the model, plays a critical role in the overall development, as does the customer, who often shapes development based on user input. Against this backdrop, there are a number of ownership and licensing questions to consider:
(1) Who owns which data?
(2) Who owns the initial and trained AI models?
(3) How are the various data, AI model, and any associated IP (e.g., patents, copyrights, know-how, and trade secrets) to be licensed among the parties?
How to Address Intellectual Property Challenges in SaMD
Protecting existing IP and recognizing value for development contributions require that answers to these questions to be sorted out, upfront, via intercompany development, clinical trial, manufacturing, or other agreements. These agreements must include highly-technical definitions. Those definitions must interface with sophisticated IP ownership and licensing provisions to establish, among other agreement elements, (a) the type of technology involved (e.g., what the AI model is to be used for), (b) ownership and use of data to be shared (e.g., what data is inputted into, used to train the AI model, outputted from the model, etc.), (c) risk based on which specific employees or labs will be contributing to the collaboration (e.g., individual scientists or research group names), (d) duration of the license, if any, for use of the project results (e.g., term or perpetual), and (e) compliance with privacy laws with respect to potentially sensitive data (e.g., medical records, personally identifiable information, etc.). In conjunction, each stakeholder must evaluate these agreements to ensure that their current and future uses are protected and that their contributions are recognized.
The licensing agreement should address each type of IP that each party is bringing to the table. First, background IP may be owned by each stakeholder but licensed (using the defined terms) narrowly as needed for collaboration or broadly. Second, independent IP may be owned by each stakeholder but deployed during the course of collaboration, and may be licensed as needed for the collaboration. It should be noted that IP developed using other parties’ IP is not “independent” in nature. Third, the new (foreground) IP should be owned by the appropriate party and licensed to others as needed for the collaboration. When possible, joint ownership should be avoided, because joint ownership may cause a myriad of problems down the line (e.g., who will handle prosecution and can enforce a patent).
In addition, all stakeholders should understand the basis for ongoing royalty payments under the license agreement, if any. One option for royalties is to make them payable for use of AI model and any associated data, including the training and output data in the scenario outlined above. Another potential option is to define royalties as being payable for products that are covered by IP, including trade secrets as well as pending and issued patents. There may also be additional considerations for royalties in the use of proprietary data.
These and other considerations should be factored when formulating IP licensing strategies in developing SaMD.
SaMD Series
For additional resources on how software as a medical device will impact the world of health care, click here to read the other articles in our series.
Foley is here to help you address all your questions and concerns relating to the use of Software as a Medical Device (SaMD). Our team of dedicated attorneys have the experience assisting clients from start-ups to publicly traded companies with respect to research, development and commercialization of SaMD products and services. Please reach out to the authors, your Foley relationship partner, our Medical Device Area of Focus or our Health Care Practice Group with any questions.


